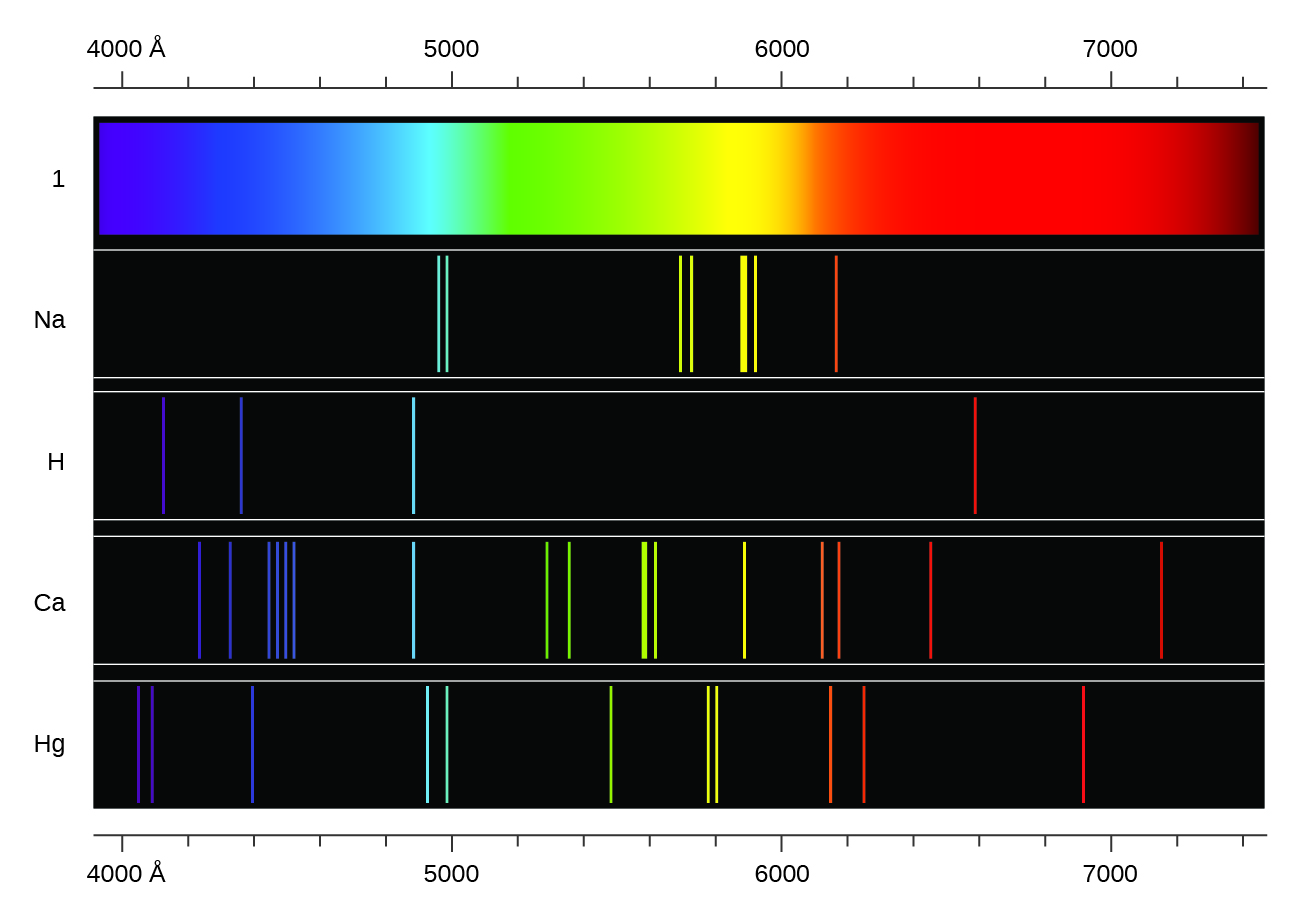
Emission Spectrum Example . Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Atoms, molecules, and ions that have absorbed. Blackbody radiation occurs when an object has a temperature,. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web halogen light bulbs emit light due to blackbody radiation. Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an.
from wisc.pb.unizin.org
Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Blackbody radiation occurs when an object has a temperature,. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web halogen light bulbs emit light due to blackbody radiation. Atoms, molecules, and ions that have absorbed. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of.
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/
Emission Spectrum Example Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Atoms, molecules, and ions that have absorbed. Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Web halogen light bulbs emit light due to blackbody radiation. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Blackbody radiation occurs when an object has a temperature,.
From chem1.com
Light, particles and waves Emission Spectrum Example Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Web halogen light bulbs emit light due to blackbody radiation. Atoms, molecules, and ions that have absorbed. Blackbody radiation occurs when an object has a temperature,. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in.. Emission Spectrum Example.
From wisc.pb.unizin.org
Day 2 Atomic Spectra and Atomic Orbitals Chemistry 109, Fall 2020 Emission Spectrum Example Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Blackbody radiation occurs when an object has a temperature,. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web halogen light bulbs emit light due to blackbody radiation. Web the emission spectrum (or line. Emission Spectrum Example.
From stock.adobe.com
Vetor de Continuous spectrum, an emission spectrum that consists of Emission Spectrum Example Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web halogen light bulbs emit light due to blackbody radiation. Atoms, molecules, and ions that have absorbed. Web in general, an. Emission Spectrum Example.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectrum Example Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Atoms, molecules, and ions that have absorbed. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum. Emission Spectrum Example.
From socratic.org
What is line emission spectrum? + Example Emission Spectrum Example Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Web halogen light bulbs emit light due to blackbody radiation. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectrum Example.
From www.visionlearning.com
History of Earth's Atmosphere I Earth Science Visionlearning Emission Spectrum Example Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Atoms, molecules, and ions that have absorbed. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web the emission spectrum (or line spectrum) of a chemical. Emission Spectrum Example.
From www.youtube.com
15 Sample Emission Spectrum YouTube Emission Spectrum Example Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web halogen light bulbs emit light due to blackbody radiation. Atoms, molecules, and ions that have absorbed. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of.. Emission Spectrum Example.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectrum Example Atoms, molecules, and ions that have absorbed. Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web learn what is emission spectrum, how it differs from absorption spectrum, and how. Emission Spectrum Example.
From ibsen.com
Fluorescense spectrum Ibsen Photonics Emission Spectrum Example Blackbody radiation occurs when an object has a temperature,. Web halogen light bulbs emit light due to blackbody radiation. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Atoms, molecules, and ions that have absorbed. Web an atomic emission spectrum is the pattern of lines formed when light passes through a. Emission Spectrum Example.
From brainly.com
What is an emission spectrum? Use the Bohr model to explain why the Emission Spectrum Example Blackbody radiation occurs when an object has a temperature,. Atoms, molecules, and ions that have absorbed. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Web the emission spectrum is the. Emission Spectrum Example.
From fity.club
Spectrum Regions Diagram Emission Spectrum Example Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Blackbody. Emission Spectrum Example.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements Webb Emission Spectrum Example Atoms, molecules, and ions that have absorbed. Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web the emission spectrum is the spectrum of radiation emitted by a substance. Emission Spectrum Example.
From www.anisotropela.dk
Redshift Emission Spectrum Example Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Blackbody radiation occurs when an object has a temperature,. Web an atomic emission spectrum is the pattern of lines formed when. Emission Spectrum Example.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectrum Example Web learn what is emission spectrum, how it differs from absorption spectrum, and how to observe it in. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it. Emission Spectrum Example.
From xmphysics.com
17.3.2 Emission Spectrum xmPhysics Emission Spectrum Example Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Web halogen light bulbs emit light due to blackbody radiation. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Atoms, molecules, and ions that have absorbed. Blackbody radiation. Emission Spectrum Example.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectrum Example Web in general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an. Blackbody radiation occurs when an object has a temperature,. Web the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Web the emission spectrum is the spectrum of radiation emitted by a substance. Emission Spectrum Example.
From www.researchgate.net
Example optical emission spectrum (top image) as well as analysis of Emission Spectrum Example Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Atoms, molecules, and ions that have absorbed. Web halogen light bulbs emit light due to blackbody radiation. Blackbody. Emission Spectrum Example.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectrum Example Atoms, molecules, and ions that have absorbed. Web the emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Web halogen light bulbs emit light due to blackbody radiation. Blackbody radiation occurs when an object has a temperature,. Web an atomic emission spectrum is the pattern of lines formed when light passes through a prism. Emission Spectrum Example.